CBSE Class 9 Science Chapter 4 Notes Structure of the Atom
Structure of the Atom Class 9 Notes Understanding the Lesson
1. Discovery of electron: Study of cathode rays.
- On electrical discharge through gases at very low pressure, cathode rays are produced.
- Cathode rays move in straight line.
- Cathode rays have some mechanical energy.
- Cathode rays consist of negatively charged particles, i.e., electrons.
2. Electron: An electron is the sub-atomic or fundamental particle which carries one unit negative charge.
It is represented by e–.
Mass of one electron = 9.11 x 10-31 kg.
Charge of one electron = – 1.6 x 10-19 C.
3. Proton: Discovered by Goldstein (1886) anode ray or canal ray experiment.
Mass of proton = 1.67 x 10-24 kg.
One proton is 1840 times heavier than electron.
Charge on proton = + 1.6 x 10-19 C.
4. Thomson’s model of an atom
He proposed that:
- An atom consists of a uniform sphere of positive electricity in which the electrons are distributed more or less uniformly.
- The negative and the positive charge are equal in magnitude. Thus, the atom as a whole is electrically neutral.
5. Rutherford’s model of an atom
Rutherford observed that:
- Most of the a-particles (nearly 99%) passed through the gold foil undeflected.
- Some of the a-particles (about one in every 20,000) were deflected by small angles.
- A few particles (1 in about 106) were either deflected by very large angles or were actually reflected back along their path.
In order to explain the observation of his scattering experiment, Rutherford assumed that the solid gold foil consists of layers of individual atoms touching each other so that there is hardly any empty space between them. Rutherford explained his observation as follows:
- Most of the space inside the atom is empty because most of the a-particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space
- A very small fraction of a-particles was deflected by 180°, indicating that all the positive charge and mass of the gold atom were, concentrated in a very small volume within the atom.
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
- There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
- The electrons revolve around the nucleus in circular paths.
- The size of the nucleus is very small as compared to the size of the atom.
6. Drawbacks of Rutherford’s model of the atom
Rutherford’s model could not explain the stability of the atom. This is because according to Rutherford’s model, an atom consists of a small heavy positively charged nucleus in the centre and the electrons revolve around it.
However, whenever a charged particle like an electron revolves around a central force like that of a nucleus, it loses energy continuously in the form of radiations. Thus, the orbit of the revolving electron will keep on becoming smaller and smaller and ultimately the electron should fall into the nucleus.
7. Discovery of Neutrons
Neutron may be defined as subatomic particle which had no charge and a mass nearly equal to that of proton:
- Mass of neutron = 1.676 x 10-24
- Charge of neutron = 0 (zero).
8. Bohr’s atomic model
- Electron revolves around nucleus only in certain selected circular orbits with definite energies and are called energy shells or energy levels.
- While revolving around the nucleus in an orbit, an electron does not lose energy nor does it gain energy.
- Different shells or orbits are numbered as 1, 2, 3, 4…………………… or designated as K, L, M, N……………….
- Every orbit is associated with a fixed amount of energy, so on gaining a certain amount of energy e~, jumps to the higher orbit.
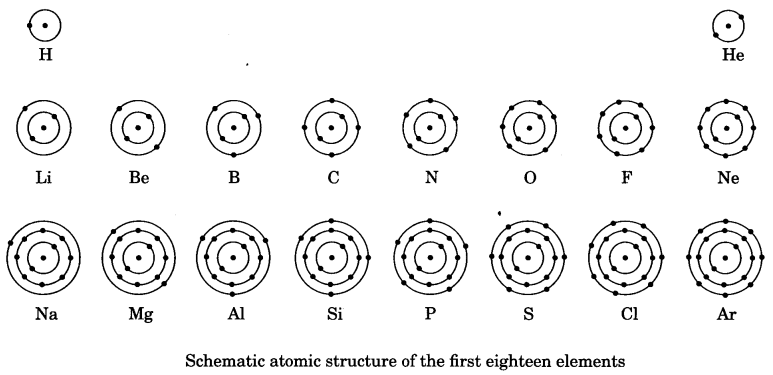
9. How are electrons distributed in different orbit (shells)?
Answer:
The distribution of the electrons in the shells is known as electronic configuration. It is based on certain guidelines or rules given by Bohr and Bury. This is known as Bohr-Bury scheme. According to this scheme,
1. The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number of energy level index, 1, 2. 3,………………… Hence the maximum number of electrons in different shells are as follows:
- First orbit or K-shell will be = 2 x 12 = 2,
- Second orbit or L-shell will be = 2 x 22 = 4,
- Third orbit or M-shell will be = 2 x 32 = 18,
- Fourth orbit or N-shell will be = 2 x 42 = 32 and so on.
3. The maximum number of electrons that can be accommodated in the outermost orbit is 8.
4. Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a step-wise manner.
Atomic structure of first eighteen elements is shown schematically in the figure given below.
10. Valency
The electrons present in the outermost shell of an atom are known as the valence electrons.
From the Bohr-Bury scheme, the outermost shell of the an atom can accommodate a maximum of 8 electrons. The combining capacity or valency of elements having a completely filled outermost shell is zero. Inert elements, like helium atom has two electrons in its outermost shell and all other elements have atoms with eight electrons in their outermost shell.
The combining capacity of the atoms of other elements was explained in terms of their tendency to attain a fully-filled outermost shell (stable octect or dulpet). The atoms which do not have their outermost shell fully filled enter into bond formation with other atoms in order to achieve an octet (or duplet) of electrons in their outermost shells. They do so either by sharing, losing or gaining electrons.
If the number of electrons in the outermost shell of an atom is close to its full capacity, then valency is determined in a different way.
11. Atomic number (Z)
The number of unit positive charges present in the nucleus of an atom is known as atomic number of the element.
It is denoted by the symbol Z.
Atomic no. (Z) = No. of protons = No. of electrons.
For example: Hydrogen, Z = 1 because in hydrogen atom, only one proton is present in the nucleus.
Similarly, for carbon Z = 6.
12. Mass number
The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. For example, mass of carbon is 12u because it has 6 protons and 6 neutrons, 6u + 6u = 12u. Similarly, the mass of aluminium is 27u because it has 13 protons and 14 neutrons.
The atomic number, mass number and symbol of the element are to be written as:
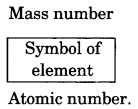
For example: Nitrogen is written as \({ }_{7}^{14} \mathrm{N}\).
13. Isotopes
The different atoms of the same element having same atomic number but different mass numbers.
For example: Hydrogen atom, has three isotopes, namely protium \(\left({ }_{1}^{1} \mathrm{H}\right)\), deuterium and tritium \(\left({ }_{1}^{3} \mathrm{H} \text { or } \mathrm{T}\right)\)
14. Applications of isotopes:
- An isotope of Uranium (U-235) is used as a fuel in nuclear reactors.
- An isotope of cobalt (C-60) is used in treatment of cancer by radiation therapy.
- An isotope of iodine is used in the treatment of goitre.
- Ages of old wooden articles are determined by observing the radioactivity of C-14 isotope of carbon.
15. Isobars:
Atoms of different elements with different atomic numbers which have the same mass number are known as isobars.
For example: Two elements – calcium, atomic number 20, and argon, atomic number 18 are isobars. The number of electrons in these atoms is different, but the mass number of both of these elements is 40.
Class 9 Science Chapter 4 Notes Important Terms
Cathode rays: It consist of negatively charged particles (electrons) emitted by passing electricity through gases at low pressure.
Anode rays: These rays are produced along with cathode rays and move towards cathode. They consist of positively charged ions.
Electrons: Electrons are fundamental particles carrying a unit negative charge and are a common constituent of all atoms.
Protons: (This positively charged particle was characterised in 1919). The fundamental particle which carries one unit of positive charge and has a mass nearly equal to that of an H-atom. Mass of proton = 1.6726 x 10-24 g.
Neutron: The fundamental particle, which has a mass nearly equal to that of an H atom but has no charge.
Mass of neutron = 1.6749 x 10-24 g.
Valency: It is the capacity of atoms of a given element to combine with, or replace atoms of hydrogen. In HCl gas, valency of chlorine is 1.
Nucleons: Protons and neutrons together are known as nucleons.
Isobars: Atoms having the same mass number but different atomic numbers.
For example, K – 40 and Ar – 40.
Isotopes: Atoms with identical atomic number but different mass numbers.
For example,\({ }_{1}^{1} \mathrm{H},{ }_{1}^{2} \mathrm{H} \text { and }{ }_{1}^{3} \mathrm{H}\)